Page 393 - Instrumentation Reference Book 3E
P. 393
376 Chemical analysis: electrochemical techniques
sulfur dioxide in the paper-making industry; rent is again a linear function of the concentra-
fluoride, calcium, chloride, nitrate, and sulfur tion of the depolarizing agent.
dioxide in foodstuffs, pH in water and effluents, The sensitivity of the analyzer is sometimes
papers, textiles, leather, and foodstuffs, and cal- increased by using buffered water as the electro-
cium, chloride, fluoride, and potassium in pharma- lyte so that the cell operates at a definite pH.
ceuticals. Amperometric instruments are inherently linear
in response, but special steps have to be taken in
order to make them specific to the substance
17.8 Com m o n elect ro c h e m i c a I whose concentration is to be measured, because
analyzers other substances may act as depolarizing agents
and so interfere with the measurement. When the
interfering substances are known steps may be
17.8.1 Residual chlorine analyzer
taken to remove them.
When two dissimilar metal electrodes are Where the instrument is intended to measure
immersed in an electrolyte and connected pollutants in air or gas, the gas to be tested is
together, current will flow due to the build-up of either bubbled through a suitable cell or arranged
electrons on the more electropositive electrode. to impinge upon the surface of the liquid in the
The current will soon stop, however, owing to cell. In these cases interfering gases can be
the fact that the cell will become polarized. removed by chemical or molecular filters in the
If, however, a suitable depolarizing agent is sampling system.
added, a current will continue to flow, the mag- This form of instrument may be used to detect
nitude of which will depend upon the concentra- halogens, such as chlorine, in air, and instruments
tion and nature of the ions producing the with ranges from 0-0.5 to 0-20 ppm are available
depolarization. Thus: by choice of suitable mater- measuring with an accuracy of f2% and a sensi-
ials for the electrodes and arranging for the tivity of 0.01 ppm. By altering the electrolyte the
addition of the depolarizing agent which is in fact instrument may be changed to measure the corres-
the substance whose concentration is to be meas- ponding acid vapors, i.e., HCI, HBr, and HF. One
ured, amperometric analyzers may be made to type of instrument for measuring chlorine in
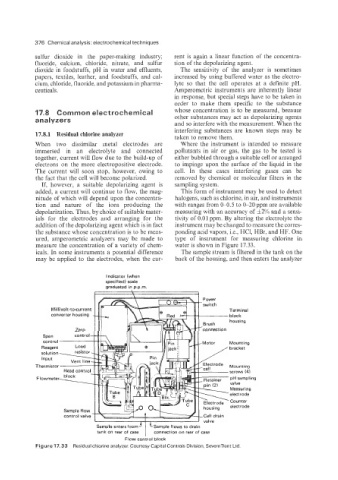
measure the concentration of a variety of chem- water is shown in Figure 17.33.
icals. In some instruments a potential difference The sample stream is filtered in the tank on the
may be applied to the electrodes, when the cur- back of the housing, and then enters the analyzer
Indicator (when
specified) scale
graduated in p.p.m.
Millivolt-to-current
converter housing
Span
control
Reagent
solution
input
Thermistor
Flowmeter
control valve
Flow control block
Figure 17.33 Residual chlorine analyzer. Courtesy Capital Controls Division, SevernTrent Ltd