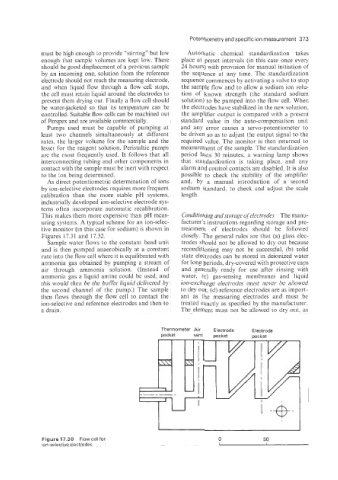
Page 390 - Instrumentation Reference Book 3E
P. 390
Potentiometry and specific ion measurement 373
must be high enough to provide “stirring” but low Automatic chemical standardization takes
enough that sample volumes are kept low. There place at preset intervals (in this case once every
should be good displacement of a previous sample 24 hours) with provision for manual initiation of
by an incoming one, solution from the reference the sequence at any time. The standardization
electrode should not reach the measuring electrode, sequence commences by activating a valve to stop
and when liquid flow through a flow cell stops, the sample flow and to allow a sodium ion solu-
the cell must retain liquid around the electrodes to tion of known strength (the standard sodium
prevent them drying out. Finally a flow cell should solution) to be pumped into the flow cell. When
be waterjacketed so that its temperature can be the electrodes have stabilized in the new solution,
controlled. Suitable flow cells can be machined out the amplifier output is compared with a present
of Perspex and are available commercially. standard value in the auto-compensation unit
Pumps used must be capable of pumping at and any error causes a servo-potentiometer to
least two channels simultaneously at different be driven so as to adjust the output signal to the
rates, the larger volume for the sample and the required value. The monitor is then returned to
lesser for the reagent solution. Peristaltic pumps measurement of the sample. The standardization
are the most frequently used. It follows that all period lasts 30 minutes, a warning lamp shows
interconnecting tubing and other components in that standardization is taking place, and any
contact with the sample must be Inert with respect alarm and control contacts are disabled. It is also
to the ion being determined. possible to check the stability of the amplifier
As direct potentiometric determination of ions and, by a manual introduction of a second
by ion-selective electrodes requires more frequent sodium standard, to check and adjust the scale
calibration than the more stable pH systems, length.
industrially developed ion-selective electrode sys-
tems often incorporate automatic recalibration.
This makes them more expensive than pH meas- Conditioning and storage of electrodes The manu-
uring systems. A typical scheme for an ion-selec- facturer’s instructions regarding storage and pre-
tive monitor (in this case for sodium) is shown in treatment of electrodes should be followed
Figures 17.31 and 17.32. closely. The general rules are that (a) glass elec-
Sampl’e water flows to the constant head unit trodes should not be allowed to dry out because
and is then pumped anaerobically at a constant reconditioning may not be successful, (b) solid
rate into the flow cell where it is equilibrated with state electrodes can be stored in deionized water
ammonia gas obtained by pumping a stream of for long periods, dry-covered with protective caps
air thro’ugh ammonia solution. (Instead of and generally ready for use after rinsing with
ammonia gas a liquid amine could be used, and water, (c) gas-sensing membranes and liquid
this would then be the buffer liquid delivered by ion-exchange electrodes must never be allowed
the second channel of the pump.) The sample to dry out, (d) reference electrodes are as import-
then flows through the flow cell to contact the ant as the measuring electrodes and must be
ion-selective and reference electrodes and then to treated exactly as specified by the manufacturer.
a drain. The element must not be allowed to dry out. as
Thermometer Air Electrode E I ectrode
pocket vent pocket pocket
I
Figurel7.30 Flow cell for 0 50
ion-selective electrodes. I I