Page 574 - Instrumentation Reference Book 3E
P. 574
556 Measurements employing nuclear techniques
counter with the scintillator of cerium-activated changes in the carbon-hydrogen ratio of the
lithium glass. As such a detector is sensitive to hydrocarbon. When the X-rays used have an
both neutrons and gamma rays, the same probe energy of 22 keV the mass attenuation for car-
may be used to detect both slowed-down neu- bon and hydrogen are equal. Thus by using
trons and gamma rays from the source-the X-rays produced by allowing the radiation from
two can be distinguished because they give pulses the radio-element ’“Am to produce fluorescent
of different shapes. This avoids the necessity of excitation in a silver target which gives X-rays
having two probes, one to measure neutrons and having an energy of 23keV, the absorption is
the other to measure scattered gammas, allowing made independent of the carbon-hydrogen
the measurement of both moisture and density by ratio. As this source has a half-life of 450yr.
pulse-shape analysis. no drift occurs owing to decay of the source.
Surface-neutron gauges are also available. in The X-rays are passed through a measuring cell
which both radioactive source and detector are through which the hydrocarbon flows and, as
mounted in a rectangular box which is simply the absorption per unit weight of sulfur is many
placed on a flat soil surface. It is important to times greater than the absorption of carbon and
provide a smooth flat surface for such measure- hydrogen, the fraction of the X-rays absorbed is
ments, as gaps cause appreciable errors in the a measure of the concentration of the sulfur
response. present. Unfortunately the degree of absorption
of X-rays is also affected by the density of the
23.2.4 Measurement of sulfur contents of liquid sample and by the concentration of trace elem-
ents of high atomic weight and of water.
hydrocarbons
The concentration of X-rays is measured by a
Sulfur occurs in many crude oils at a concentra- high-resolution proportional counter so the accur-
tion of up to 5 percent by weight and persists acy will be a function of the statistical variation in
to a lesser extent in the refined product. As the count rate and the stability of the detector
legislation in many countries prohibits the burn- and associated electronics, which can introduce
ing of ftiels with a high sulfur content to min- an error of &0.01 per cent sulfur. A water content
imize pollution, and sulfur compounds corrode of 650ppm will also introduce an error of 0.01
engines and boilers and inhibit catalysts, it is percent sulfur. Compensation for density varia-
essential to reduce the concentration to toler- tions may be achieved by measuring the density
able levels. Thus rapid measurement of sulfur with a non-nucleonic meter and electronically
content is essential, and the measurement of the correcting the sulfur signal.
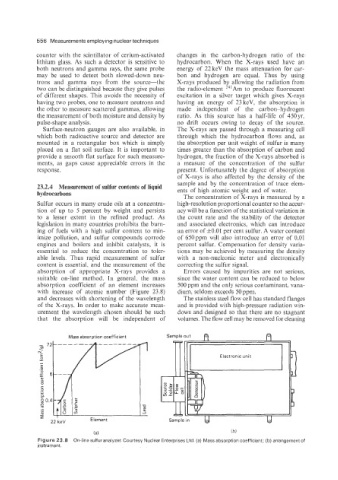
absorption of appropriate X-rays provides a Errors caused by impurities are not serious,
suitable on-line method. In general, the mass since the water content can be reduced to below
absorption coefficient of an element increases 500 ppm and the only serious contaminant, vana-
with increase of atomic number (Figure 23.8) dium. seldom exceeds 50 ppm.
and decreases with shortening of the wavelength The stainless steel flow cell has standard flanges
of the X-rays. In order to make accurate meas- and is provided with high-pressure radiation win-
urement the wavelength chosen should be such dows and designed so that there are no stagnant
that the absorption will be independent of volumes. The flow cell may be removed for cleaning
Mass absorption coefficient
N
Electronic unit
22 keV Element
Figure 23.8 On-line sulfur analyzer. Courtesy Nuclear Enterprises Ltd. (a) Mass absorption coefficient; (b) arrangement of
instrument.