Page 322 - Book Hosokawa Nanoparticle Technology Handbook
P. 322
5.4 NANOPORE CHARACTERIZATION FUNDAMENTALS
140 [17] K. Nogi, Y. Okada and K. Ogino: Mat. Trans., JIM, 35
C (√31×√31) R ± 9° (3), 156–160 (1994).
130 A [18] B.B. Pate: Surf. Sci., 165, 83–142 (1986).
R C (1×1) [19] K. Nogi: Materia, 35 (5), 523–525 (1996).
Contact angle (°) 120 [20] P. Shen, H. Fujii and K. Nogi: Scripta Mater., 52,
1259–1263 (2005).
110
[21] P. Shen, H. Fujii and K. Nogi: J. Mater. Res., 20(4),
100
Apr (2005).
90 [22] P. Shen, H. Fujii, T. Matsumoto and K. Nogi, J. Am.
Ceram. Soc., 88 (4), 912–917 (2005).
80 [23] B.B. Pate, P.M. Tefan, C. Binns, P.J. Jupiter, M.L.
Sheck, I. Lindau and W.E. Spicer: J. Vac. Sci. Technol.,
70
700 800 900 1000 1100 1200 1300 1400 1500 1600 A, 19, 349–354 (1981).
Temperature (°C) [24] V. Hamza, G.D. Kubiak and R.H. Stulen: Surf. Sci.,
206, L833–L844 (1988).
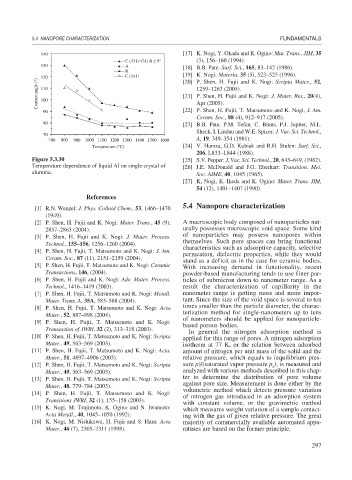
Figure 5.3.30 [25] S.V. Pepper: J. Vac. Sci. Technol., 20, 643–649, (1982).
Temperature dependence of liquid Al on single crystal of [26] J.E. McDonald and J.G. Eberhart: Transition. Met.
alumina. Soc. AIME, 40, 1045 (1965).
[27] K, Nogi, K. Ikeda and K. Ogino: Mater. Trans. JIM,
54 (12), 1401–1407 (1990).
References
5.4 Nanopore characterization
[1] R.N. Wenzel: J. Phys. Colloid Chem., 53, 1466–1470
(1949).
[2] P. Shen, H. Fujii and K. Nogi: Mater. Trans., 45 (9), A macroscopic body composed of nanoparticles nat-
2857–2863 (2004). urally possesses microscopic void space. Some kind
of nanoparticles may possess nanopores within
[3] P. Shen, H. Fujii and K. Nogi: J. Mater. Process.
themselves. Such pore spaces can bring functional
Technol., 155–156, 1256–1260 (2004).
characteristics such as adsorptive capacity, selective
[4] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: J. Am.
permeation, dielectric properties, while they would
Ceram. Soc., 87 (11), 2151–2159 (2004).
stand as a deficit as in the case for ceramic bodies.
[5] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Ceramic With increasing demand in functionality, recent
Transactions, 146, (2004). powder-based manufacturing tends to use finer par-
[6] P. Shen, H. Fujii and K. Nogi: Adv. Mater. Process. ticles of submicron down to nanometer range. As a
Technol., 1416–1419 (2003). result the characterization of capillarity in the
[7] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Metall. nanometer range is getting more and more impor-
Mater. Trans. A, 35A, 583–588 (2004). tant. Since the size of the void space is several to ten
times smaller than the particle diameter, the charac-
[8] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Acta
terization method for single-nanometers up to tens
Mater., 52, 887–898 (2004).
of nanometers should be applied for nanoparticle-
[9] P. Shen, H. Fujii, T. Matsumoto and K. Nogi:
based porous bodies.
Transaction of JWRI, 32 (2), 313–318 (2003).
In general the nitrogen adsorption method is
[10] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Scripta applied for this range of pores. A nitrogen adsorption
Mater., 49, 563–569 (2003). isotherm at 77 K, or the relation between adsorbed
[11] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Acta. amount of nitrogen per unit mass of the solid and the
Mater., 51, 4897–4906 (2003). relative pressure, which equals to (equilibrium pres-
[12] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Scripta sure p)/(saturated vapor pressure p ), is measured and
s
Mater., 49, 563–569 (2003). analyzed with various methods described in this chap-
ter to determine the distribution of pore volume
[13] P. Shen, H. Fujii, T. Matsumoto and K. Nogi: Scripta
against pore size. Measurement is done either by the
Mater., 48, 779–784 (2003).
volumetric method which detects pressure variation
[14] P. Shen, H. Fujii, T. Matsumoto and K. Nogi:
of nitrogen gas introduced in an adsorption system
Transitions JWRI, 32 (1), 155–158 (2003).
with constant volume, or the gravimetric method
[15] K. Nogi, M. Tsujimoto, K. Ogino and N. Iwamoto: which measures weight variation of a sample contact-
Acta Metall., 40, 1045–1050 (1992). ing with the gas of given relative pressure. The great
[16] K. Nogi, M. Nishikawa, H. Fujii and S. Hara: Acta majority of commercially available automated appa-
Mater., 46 (7), 2305–2311 (1998). ratuses are based on the former principle.
297