Page 320 - Book Hosokawa Nanoparticle Technology Handbook
P. 320
5.3 SURFACE STRUCTURE FUNDAMENTALS
system is a wetting or a non-wetting one since many Table 5.3.3
factors affect the wettability. Hydrogen desorption temperature on diamond surface
Relation between contact angle and surface free [18–21].
energy of solid, surface free energy of liquid (surface
tension), and interfacial free energy can be expressed Surface orientation Desorption temperature (K)
by an equation (5.3.7), which is so-called Young’s (1 0 0) 1,073
equation:
(1 1 0) 1,173, 1,223
(1 1 1) 1,273
cos sl (5.3.7)
l
s
where is the contact angle of liquid drop on a
smooth surface of solid, is the surface free energy
l
(surface tension) of liquid, is the surface free
s
energy of solid, and is the interfacial free energy
sl
between solid and liquid.
The work of adhesion, W , which is defined as a
ad,
reversible work to separate liquid from solid, is
expressed by an equation (5.3.8).
W l
(1 cos ) (5.3.8)
ad
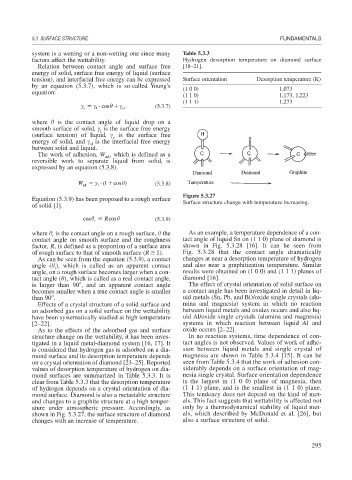
Figure 5.3.27
Equation (5.3.9) has been proposed to a rough surface Surface structure change with temperature increasing.
of solid [1].
cos R cos (5.3.9)
r
where is the contact angle on a rough surface, the As an example, a temperature dependence of a con-
r
contact angle on smooth surface and the roughness tact angle of liquid Sn on (1 1 0) plane of diamond is
factor, R, is defined as a proportion of a surface area shown in Fig. 5.3.28 [16]. It can be seen from
of rough surface to that of smooth surface (R 1). Fig. 5.3.28 that the contact angle dramatically
As can be seen from the equation (5.3.9), a contact changes at near a desorption temperature of hydrogen
angle ( ), which is called as an apparent contact and also near a graphitization temperature. Similar
r
angle, on a rough surface becomes larger when a con- results were obtained on (1 0 0) and (1 1 1) planes of
tact angle ( ), which is called as a real contact angle, diamond [16].
is larger than 90 , and an apparent contact angle The effect of crystal orientation of solid surface on
becomes smaller when a true contact angle is smaller a contact angle has been investigated in detail in liq-
than 90 . uid metals (Sn, Pb, and Bi)/oxide single crystals (alu-
Effects of a crystal structure of a solid surface and mina and magnesia) system in which no reaction
an adsorbed gas on a solid surface on the wettability between liquid metals and oxides occurs and also liq-
have been systematically studied at high temperature uid Al/oxide single crystals (alumina and magnesia)
[2–22]. systems in which reaction between liquid Al and
As to the effects of the adsorbed gas and surface oxide occurs [2–22].
structure change on the wettability, it has been inves- In no reaction systems, time dependence of con-
tigated in a liquid metal-diamond system [16, 17]. It tact angles is not observed. Values of work of adhe-
is considered that hydrogen gas is adsorbed on a dia- sion between liquid metals and single crystal of
mond surface and its desorption temperature depends magnesia are shown in Table 5.3.4 [15]. It can be
on a crystal orientation of diamond [23–25]. Reported seen from Table 5.3.4 that the work of adhesion con-
values of desorption temperature of hydrogen on dia- siderably depends on a surface orientation of mag-
mond surfaces are summarized in Table 5.3.3. It is nesia single crystal. Surface orientation dependence
clear from Table 5.3.3 that the desorption temperature is the largest in (1 0 0) plane of magnesia, then
of hydrogen depends on a crystal orientation of dia- (1 1 1) plane, and is the smallest in (1 1 0) plane.
mond surface. Diamond is also a metastable structure This tendency does not depend on the kind of met-
and changes to a graphite structure at a high temper- als. This fact suggests that wettability is affected not
ature under atmospheric pressure. Accordingly, as only by a thermodynamical stability of liquid met-
shown in Fig. 5.3.27, the surface structure of diamond als, which described by McDonald et al. [26], but
changes with an increase of temperature. also a surface structure of solid.
295