Page 323 - Book Hosokawa Nanoparticle Technology Handbook
P. 323
FUNDAMENTALS CH. 5 CHARACTERIZATION METHODS FOR NANOSTRUCTURE OF MATERIALS
In the following sections, various methods for ana- type, and the capillary condensation phenomenon is
lyzing nitrogen isotherms to determine pore size dis- for the latter.
tribution (PSD) will be described with its emphasis on
nanometer range. Some other experimental methods 5.4.2 Micropore filling phenomenon and PSD analysis
are briefly explained in the last part of this section.
5.4.2.1 Micropore filling
5.4.1 Type of nitrogen isotherms and pore Within the space of narrow pores in the range of 1 or
characteristics implied 2 nm, the interaction potential energies of opposite sur-
faces will overlap significantly, resulting in a strongly
The International Union of Pure and Applied attractive field as shown in Fig. 5.4.2 for slit-pore case.
Chemistry (IUPAC) established classification of pore Adsorbate molecules are attracted and trapped into the
size into the following three categories. space by this strong field to form up a lump of mole-
Macropore: Greater than 50 nm. cules with the entropy state similar to that of liquid,
Mesopore: 2–50 nm. which differs significantly from monolayer adsorption
Micropore: Smaller than 2 nm. phenomenon of covering solid surface.
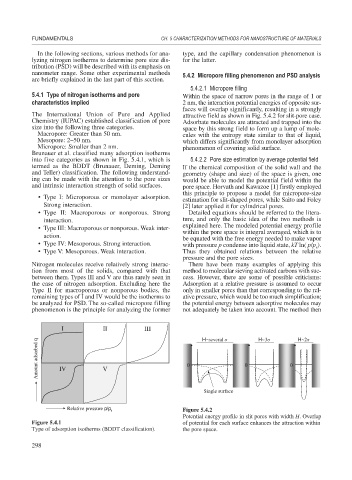
Brunauer et al. classified many adsorption isotherms
into five categories as shown in Fig. 5.4.1, which is 5.4.2.2 Pore size estimation by average potential field
termed as the BDDT (Brunauer, Deming, Deming If the chemical composition of the solid wall and the
and Teller) classification. The following understand- geometry (shape and size) of the space is given, one
ing can be made with the attention to the pore sizes would be able to model the potential field within the
and intrinsic interaction strength of solid surfaces. pore space. Horvath and Kawazoe [1] firstly employed
this principle to propose a model for micropore-size
• Type I: Microporous or monolayer adsorption.
estimation for slit-shaped pores, while Saito and Foley
Strong interaction. [2] later applied it for cylindrical pores.
• Type II: Macroporous or nonporous. Strong Detailed equations should be referred to the litera-
interaction. ture, and only the basic idea of the two methods is
explained here. The modeled potential energy profile
• Type III: Macroporous or nonporous. Weak inter-
within the pore space is integral averaged, which is to
action.
be equated with the free energy needed to make vapor
• Type IV: Mesoporous. Strong interaction. with pressure p condense into liquid state, kT ln( p/p ).
s
• Type V: Mesoporous. Weak interaction. Thus they obtained relations between the relative
pressure and the pore sizes.
Nitrogen molecules receive relatively strong interac- There have been many examples of applying this
tion from most of the solids, compared with that method to molecular sieving activated carbons with suc-
between them. Types III and V are thus rarely seen in cess. However, there are some of possible criticisms:
the case of nitrogen adsorption. Excluding here the Adsorption at a relative pressure is assumed to occur
Type II for macroporous or nonporous bodies, the only in smaller pores than that corresponding to the rel-
remaining types of I and IV would be the isotherms to ative pressure, which would be too much simplification;
be analyzed for PSD. The so-called micropore filling the potential energy between adsorptive molecules may
phenomenon is the principle for analyzing the former not adequately be taken into account. The method then
I II III H~several H~3 H~2
Amount adsorbed q IV V 0 0 0
Single surface
Relative pressure p/p s Figure 5.4.2
Potential energy profile in slit pores with width H. Overlap
Figure 5.4.1 of potential for each surface enhances the attraction within
Type of adsorption isotherms (BDDT classification). the pore space.
298