Page 325 - Book Hosokawa Nanoparticle Technology Handbook
P. 325
FUNDAMENTALS CH. 5 CHARACTERIZATION METHODS FOR NANOSTRUCTURE OF MATERIALS
above two equations. The apparent difference of
adsorption volume between the two points is to be
corrected by subtracting the variation of t in pores
larger than the representative pore size, which should
give the pore volume for the pore size. Iteration of this
process gives the PSD.
Many of detailed calculation schemes were pro-
posed by, e.g., Cranston and Inkley [5], Dollimore and
Heal [6], and Barell et al. [7], at least one of which is
installed in a commercial apparatus as a PSD soft-
ware. Major difference between these methods is the
data or equation for adsorption thickness t, and the
schemes of calculation themselves do not produce
significant difference if common data for t are used.
Therefore one need not worry which method is
installed in one’s commercial apparatus, but should
pay more attention to the t-data or standard isotherms
in the software; how precise and how abundant for
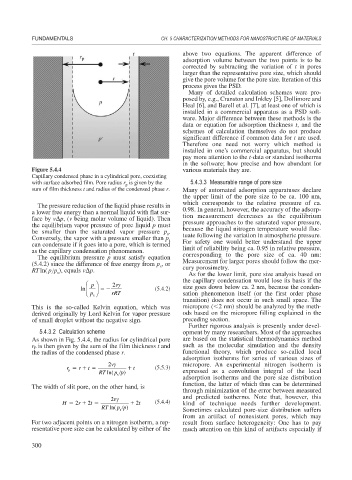
Figure 5.4.4 various materials they are.
Capillary condensed phase in a cylindrical pore, coexisting
with surface adsorbed film. Pore radius r is given by the 5.4.3.3 Measurable range of pore size
p
sum of film thickness t and radius of the condensed phase r. Many of automated adsorption apparatuses declare
the upper limit of the pore size to be ca. 100 nm,
which corresponds to the relative pressure of ca.
The pressure reduction of the liquid phase results in
a lower free energy than a normal liquid with flat sur- 0.98. In general, however, the accuracy of the adsorp-
face by v p, (v being molar volume of liquid). Then tion measurement decreases as the equilibrium
the equilibrium vapor pressure of pore liquid p must pressure approaches to the saturated vapor pressure,
be smaller than the saturated vapor pressure p . because the liquid nitrogen temperature would fluc-
s
Conversely, the vapor with a pressure smaller than p s tuate following the variation in atmospheric pressure.
can condensate if it goes into a pore, which is termed For safety one would better understand the upper
as the capillary condensation phenomenon. limit of reliability being ca. 0.95 in relative pressure,
The equilibrium pressure p must satisfy equation corresponding to the pore size of ca. 40 nm:
(5.4.2) since the difference of free energy from p , or Measurement for larger pores should follow the mer-
s
RTln( p p ), equals v p. cury porosimetry.
s As for the lower limit, pore size analysis based on
⎛ p ⎞ 2 the capillary condensation would lose its basis if the
ln ⎜ ⎟ (5.4.2) size goes down below ca. 2 nm, because the conden-
⎝ p ⎠ rRT sation phenomenon itself (or the first order phase
s
transition) does not occur in such small space. The
This is the so-called Kelvin equation, which was micropore ( 2 nm) should be analyzed by the meth-
derived originally by Lord Kelvin for vapor pressure ods based on the micropore filling explained in the
of small droplet without the negative sign. preceding section.
Further rigorous analysis is presently under devel-
5.4.3.2 Calculation scheme opment by many researchers. Most of the approaches
As shown in Fig. 5.4.4, the radius for cylindrical pore are based on the statistical thermodynamics method
rp is then given by the sum of the film thickness t and such as the molecular simulation and the density
the radius of the condensed phase r. functional theory, which produce so-called local
adsorption isotherms for series of various sizes of
2 micropore. An experimental nitrogen isotherm is
r t (5.5.3)
r
t
RT ln( expressed as a convolution integral of the local
p
p p)
s
adsorption isotherms and the pore size distribution
function, the latter of which thus can be determined
The width of slit pore, on the other hand, is
through minimization of the error between measured
2 and predicted isotherms. Note that, however, this
H 2 r 2 t t 2 (5.4.4) kind of technique needs further development.
RT ln(
p p)
s Sometimes calculated pore-size distribution suffers
from an artifact of nonexistent pores, which may
For two adjacent points on a nitrogen isotherm, a rep- result from surface heterogeneity: One has to pay
resentative pore size can be calculated by either of the much attention on this kind of artifacts especially if
300