Page 317 - Book Hosokawa Nanoparticle Technology Handbook
P. 317
FUNDAMENTALS CH. 5 CHARACTERIZATION METHODS FOR NANOSTRUCTURE OF MATERIALS
such materials generally give rise to unwanted shifts
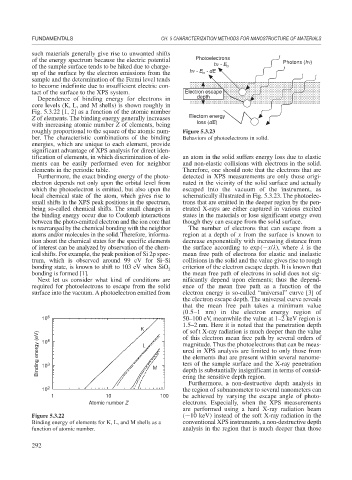
of the energy spectrum because the electric potential Photoelectrons Photons (h )
of the sample surface tends to be hiked due to charge- h - E b
up of the surface by the electron emissions from the h - E - dE
b
sample and the determination of the Fermi level tends
to become indefinite due to insufficient electric con-
tact of the surface to the XPS system. Electron escape
Dependence of binding energy for electrons in depth
core levels (K, L, and M shells) is shown roughly in
Fig. 5.3.22 [1, 2] as a function of the atomic number
Z of elements. The binding energy generally increases Electorn energy
with increasing atomic number Z of elements, being loss (dE)
roughly proportional to the square of the atomic num- Figure 5.3.23
ber. The characteristic combinations of the binding Behaviors of photoelectrons in solid.
energies, which are unique to each element, provide
significant advantage of XPS analysis for direct iden-
tification of elements, in which discrimination of ele- an atom in the solid suffers energy loss due to elastic
ments can be easily performed even for neighbor and non-elastic collisions with electrons in the solid.
elements in the periodic table. Therefore, one should note that the electrons that are
Furthermore, the exact binding energy of the photo- detected in XPS measurements are only those origi-
electron depends not only upon the orbital level from nated in the vicinity of the solid surface and actually
which the photoelectron is emitted, but also upon the escaped into the vacuum of the instrument, as
local chemical state of the atom, which gives rise to schematically illustrated in Fig. 5.3.23. The photoelec-
small shifts in the XPS peak positions in the spectrum, trons that are emitted in the deeper region by the pen-
being so-called chemical shifts. The small changes in etrated X-rays are either captured in various excited
the binding energy occur due to Coulomb interactions states in the materials or lose significant energy even
between the photo-emitted electron and the ion core that though they can escape from the solid surface.
is rearranged by the chemical bonding with the neighbor The number of electrons that can escape from a
atoms and/or molecules in the solid. Therefore, informa- region at a depth of x from the surface is known to
tion about the chemical states for the specific elements decrease exponentially with increasing distance from
of interest can be analyzed by observation of the chem- the surface according to exp( x/ ), where is the
ical shifts. For example, the peak position of Si 2p spec- mean free path of electrons for elastic and inelastic
trum, which is observed around 99 eV for Si–Si collisions in the solid and the value gives rise to rough
bonding state, is known to shift to 103 eV when SiO 2 criterion of the electron escape depth. It is known that
bonding is formed [1]. the mean free path of electrons in solid does not sig-
Next let us consider what kind of conditions are nificantly depend upon elements; thus the depend-
required for photoelectrons to escape from the solid ence of the mean free path as a function of the
surface into the vacuum. A photoelectron emitted from electron energy is so-called “universal” curve [3] of
the electron escape depth. The universal curve reveals
that the mean free path takes a minimum value
(0.5–1 nm) in the electron energy region of
10 5 50–100 eV, meanwhile the value at 1–2 keV region is
1.5–2 nm. Here it is noted that the penetration depth
K of soft X-ray radiation is much deeper than the value
Binding energy (eV) 10 3 L M magnitude. Thus the photoelectrons that can be meas-
of this electron mean free path by several orders of
4
ured in XPS analysis are limited to only those from
the elements that are present within several nanome-
ters of the sample surface and the X-ray penetration
10
depth is substantially insignificant in terms of consid-
ering the sensitive depth region.
Furthermore, a non-destructive depth analysis in
10 2 the region of subnanometer to several nanometers can
1 10 100 be achieved by varying the escape angle of photo-
Atomic number Z electrons. Especially, when the XPS measurements
are performed using a hard X-ray radiation beam
Figure 5.3.22 ( 10 keV) instead of the soft X-ray radiation in the
Binding energy of elements for K, L, and M shells as a conventional XPS instruments, a non-destructive depth
function of atomic number. analysis in the region that is much deeper than those
292