Page 567 - Book Hosokawa Nanoparticle Technology Handbook
P. 567
28 DEVELOPMENT OF FUEL CELLS APPLICATIONS
In the current power generating system such as 1. Development task of fuel cells
thermal power plant, the fuel is first made to heat,
the turbine is rotated by the heat energy, and this The fuel cell is classified into some kinds after the
energy is converted into electricity. The power gen- electrolyte used as follows: PAFC (phosphoric acid
eration efficiency of such method is normally less fuel cells), PEFC (polymer electrolyte fuel cells),
than 40% according to the restriction at Carnot MCFC (molten carbonate fuel cells) and SOFCs. In
cycle. On the other hand, because the fuel cells do PAFC and PEFC, proton (H ) moves in the elec-
not face the restriction at Carnot cycle and can trolyte. Also, in MCFC, the carbonate ion moves and
directly convert the energy of fuel into the electric- in SOFC, the oxygen ion moves in the electrolyte. Ion
ity, highly effective power generation is possible. conductivity (mobility) in the electrolyte depends on
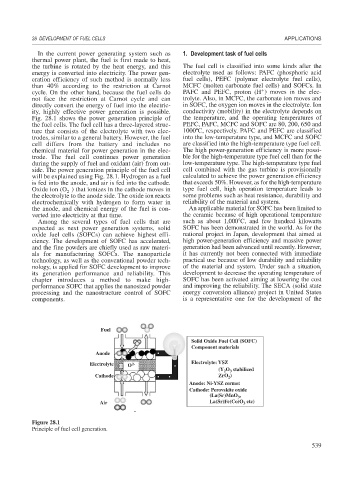
Fig. 28.1 shows the power generation principle of the temperature, and the operating temperatures of
the fuel cells. The fuel cell has a three-layered struc- PEFC, PAFC, MCFC and SOFC are 80, 200, 650 and
ture that consists of the electrolyte with two elec- 1000ºC, respectively. PAFC and PEFC are classified
trodes, similar to a general battery. However, the fuel into the low-temperature type, and MCFC and SOFC
cell differs from the battery and includes no are classified into the high-temperature type fuel cell.
chemical material for power generation in the elec- The high power-generation efficiency is more possi-
trode. The fuel cell continues power generation ble for the high-temperature type fuel cell than for the
during the supply of fuel and oxidant (air) from out- low-temperature type. The high-temperature type fuel
side. The power generation principle of the fuel cell cell combined with the gas turbine is provisionally
will be explained using Fig. 28.1. Hydrogen as a fuel calculated to achieve the power generation efficiency
is fed into the anode, and air is fed into the cathode. that exceeds 50%. However, as for the high-temperature
Oxide ion (O ) that ionizes in the cathode moves in type fuel cell, high operation temperature leads to
2
the electrolyte to the anode side. The oxide ion reacts some problems such as heat resistance, durability and
electrochemically with hydrogen to form water in reliability of the material and system.
the anode, and chemical energy of the fuel is con- An applicable material for SOFC has been limited to
verted into electricity at that time. the ceramic because of high operational temperature
Among the several types of fuel cells that are such as about 1,000 C, and few hundred kilowatts
expected as next power generation systems, solid SOFC has been demonstrated in the world. As for the
oxide fuel cells (SOFCs) can achieve highest effi- national project in Japan, development that aimed at
ciency. The development of SOFC has accelerated, high power-generation efficiency and massive power
and the fine powders are chiefly used as raw materi- generation had been advanced until recently. However,
als for manufacturing SOFCs. The nanoparticle it has currently not been connected with immediate
technology, as well as the conventional powder tech- practical use because of low durability and reliability
nology, is applied for SOFC development to improve of the material and system. Under such a situation,
its generation performance and reliability. This development to decrease the operating temperature of
chapter introduces a method to make high- SOFC has been activated aiming at lowering the cost
performance SOFC that applies the nanosized powder and improving the reliability. The SECA (solid state
processing and the nanostructure control of SOFC energy conversion alliance) project in United States
components. is a representative one for the development of the
Fuel H 2 H O load
2
Solid Oxide Fuel Cell (SOFC)
Component materials
Anode
Electrolyte O 2- Electrolyte: YSZ
(Y O stabilized
3
2
Cathode ZrO )
2
Anode: Ni-YSZ cermet
Cathode: Perovskite oxide
(La(Sr)MnO ,
3
O
Air 2 N 2 La(Sr)Fe(Co)O etc)
3
N 2
Figure 28.1
Principle of fuel cell generation.
539