Page 134 - New Trends In Coal Conversion
P. 134
Coal and biomass cofiring: CFD modeling 97
d½NO
S Thermal NOx ¼ $MW NO (4.7)
dt
thermal
in which MW NO is the molecular weight of NO.
4.3.6.2 Fuel NO x
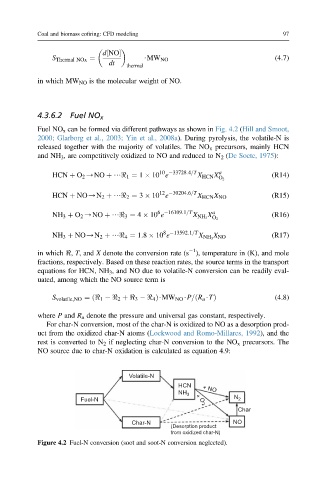
Fuel NO x can be formed via different pathways as shown in Fig. 4.2 (Hill and Smoot,
2000; Glarborg et al., 2003; Yin et al., 2008a). During pyrolysis, the volatile-N is
released together with the majority of volatiles. The NO x precursors, mainly HCN
and NH 3 , are competitively oxidized to NO and reduced to N 2 (De Soete, 1975):
10 33728:4=T a
HCN þ O 2 /NO þ /< 1 ¼ 1 10 e X HCN X (R14)
O 2
12 30204:6=T
HCN þ NO/N 2 þ /< 2 ¼ 3 10 e X HCN X NO (R15)
6 16109:1=T
a
NH 3 þ O 2 /NO þ /< 3 ¼ 4 10 e X NH 3 O 2 (R16)
X
8 13592:1=T
X
NH 3 þ NO/N 2 þ /< 4 ¼ 1:8 10 e X NH 3 NO (R17)
1
in which <, T, and X denote the conversion rate (s ), temperature in (K), and mole
fractions, respectively. Based on these reaction rates, the source terms in the transport
equations for HCN, NH 3 , and NO due to volatile-N conversion can be readily eval-
uated, among which the NO source term is
S volatile;NO ¼ð< 1 < 2 þ< 3 < 4 Þ$MW NO $P=ðR u $TÞ (4.8)
where P and R u denote the pressure and universal gas constant, respectively.
For char-N conversion, most of the char-N is oxidized to NO as a desorption prod-
uct from the oxidized char-N atoms (Lockwood and Romo-Millares, 1992), and the
rest is converted to N 2 if neglecting char-N conversion to the NO x precursors. The
NO source due to char-N oxidation is calculated as equation 4.9:
Volatile-N
HCN
+ NO
NH 3
Fuel-N + O 2 N 2
Char
Char-N NO
(Desorption product
from oxidized char-N)
Figure 4.2 Fuel-N conversion (soot and soot-N conversion neglected).