Page 156 - Semiconductor Manufacturing Handbook
P. 156
Geng(SMH)_CH12.qxd 04/04/2005 19:49 Page 12.5
PLASMA ETCHING
PLASMA ETCHING 12.5
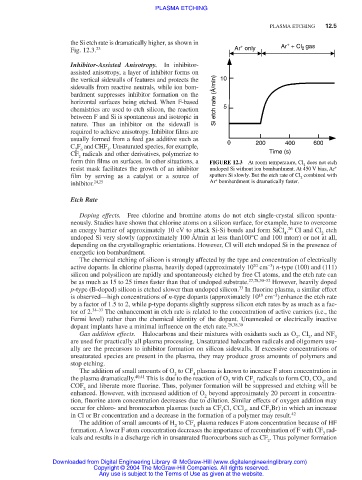
the Si etch rate is dramatically higher, as shown in + Ar + Cl gas
+
Fig. 12.3. 23 Ar only 2
Inhibitor-Assisted Anisotropy. In inhibitor-
assisted anisotropy, a layer of inhibitor forms on
the vertical sidewalls of features and protects the 10
sidewalls from reactive neutrals, while ion bom-
bardment suppresses inhibitor formation on the
horizontal surfaces being etched. When F-based Si etch rate (Å/min)
chemistries are used to etch silicon, the reaction 5
between F and Si is spontaneous and isotropic in
nature. Thus an inhibitor on the sidewall is
required to achieve anisotropy. Inhibitor films are
usually formed from a feed gas additive such as 0 200 400 600
C F and CHF . Unsaturated species, for example,
2 6 3
CF radicals and other derivatives, polymerize to Time (s)
2
form thin films on surfaces. In other situations, a FIGURE 12.3 At room temperature, Cl does not etch
2
resist mask facilitates the growth of an inhibitor undoped Si without ion bombardment. At 450 V bias, Ar +
film by serving as a catalyst or a source of sputters Si slowly. But the etch rate of Cl combined with
2
+
inhibitor. 24,25 Ar bombardment is dramatically faster.
Etch Rate
Doping effects. Free chlorine and bromine atoms do not etch single-crystal silicon sponta-
neously. Studies have shown that chlorine atoms on a silicon surface, for example, have to overcome
26
an energy barrier of approximately 10 eV to attack Si-Si bonds and form SiCl . Cl and Cl etch
4 2
undoped Si very slowly (approximately 100 Å/min at less than100°C and 100 mtorr) or not at all,
depending on the crystallographic orientations. However, Cl will etch undoped Si in the presence of
energetic ion bombardment.
The chemical etching of silicon is strongly affected by the type and concentration of electrically
−3
20
active dopants. In chlorine plasma, heavily doped (approximately 10 cm ) n-type (100) and (111)
silicon and polysilicon are rapidly and spontaneously etched by free Cl atoms, and the etch rate can
be as much as 15 to 25 times faster than that of undoped substrate. 27,28,30–32 However, heavily doped
33
p-type (B-doped) silicon is etched slower than undoped silicon. In fluorine plasma, a similar effect
19
−3
is observed—high concentrations of n-type dopants (approximately 10 cm ) enhance the etch rate
by a factor of 1.5 to 2, while p-type dopants slightly suppress silicon etch rates by as much as a fac-
tor of 2. 34–37 The enhancement in etch rate is related to the concentration of active carriers (i.e., the
Fermi level) rather than the chemical identity of the dopant. Unannealed or electrically inactive
dopant implants have a minimal influence on the etch rate. 29,38,39
Gas addition effects. Halocarbons and their mixtures with oxidants such as O , Cl , and NF
2 2 3
are used for practically all plasma processing. Unsaturated halocarbon radicals and oligomers usu-
ally are the precursors to inhibitor formation on silicon sidewalls. If excessive concentrations of
unsaturated species are present in the plasma, they may produce gross amounts of polymers and
stop etching.
The addition of small amounts of O to CF plasma is known to increase F atom concentration in
4
2
the plasma dramatically. 40,41 This is due to the reaction of O with CF radicals to form CO, CO , and
2 x 2
COF and liberate more fluorine. Thus, polymer formation will be suppressed and etching will be
2
enhanced. However, with increased addition of O beyond approximately 20 percent in concentra-
2
tion, fluorine atom concentration decreases due to dilution. Similar effects of oxygen addition may
occur for chloro- and bromocarbon plasmas (such as CF Cl, CCl , and CF Br) in which an increase
3 4 3
in Cl or Br concentration and a decrease in the formation of a polymer may result. 42
The addition of small amounts of H to CF plasma reduces F atom concentration because of HF
2 4
formation. A lower F atom concentration decreases the importance of recombination of F with CF rad-
3
icals and results in a discharge rich in unsaturated fluorocarbons such as CF . Thus polymer formation
2
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.