Page 204 - Semiconductor Manufacturing Handbook
P. 204
Geng(SMH)_CH14.qxd 04/04/2005 19:52 Page 14.5
CHEMICAL VAPOR DEPOSITION
CHEMICAL VAPOR DEPOSITION 14.5
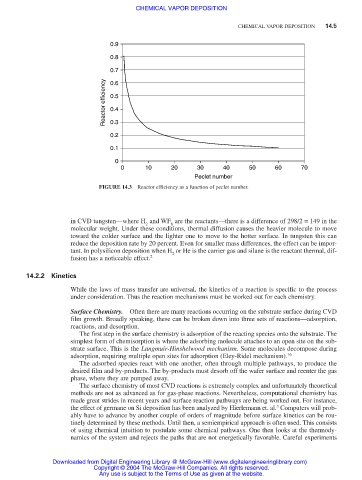
0.9
0.8
0.7
Reactor efficiency 0.5
0.6
0.4
0.3
0.2
0.1
0
0 10 20 30 40 50 60 70
Peclet number
FIGURE 14.3 Reactor efficiency as a function of peclet number.
in CVD tungsten—where H and WF are the reactants—there is a difference of 298/2 = 149 in the
2 6
molecular weight. Under these conditions, thermal diffusion causes the heavier molecule to move
toward the colder surface and the lighter one to move to the hotter surface. In tungsten this can
reduce the deposition rate by 20 percent. Even for smaller mass differences, the effect can be impor-
tant. In polysilicon deposition when H or He is the carrier gas and silane is the reactant thermal, dif-
2
fusion has a noticeable effect. 2
14.2.2 Kinetics
While the laws of mass transfer are universal, the kinetics of a reaction is specific to the process
under consideration. Thus the reaction mechanisms must be worked out for each chemistry.
Surface Chemistry. Often there are many reactions occurring on the substrate surface during CVD
film growth. Broadly speaking, these can be broken down into three sets of reactions—adsorption,
reactions, and desorption.
The first step in the surface chemistry is adsorption of the reacting species onto the substrate. The
simplest form of chemisorption is where the adsorbing molecule attaches to an open site on the sub-
strate surface. This is the Langmuir-Hinshelwood mechanism. Some molecules decompose during
adsorption, requiring multiple open sites for adsorption (Eley-Ridel mechanism). 16
The adsorbed species react with one another, often through multiple pathways, to produce the
desired film and by-products. The by-products must desorb off the wafer surface and reenter the gas
phase, where they are pumped away.
The surface chemistry of most CVD reactions is extremely complex and unfortunately theoretical
methods are not as advanced as for gas-phase reactions. Nevertheless, computational chemistry has
made great strides in recent years and surface reaction pathways are being worked out. For instance,
3
the effect of germane on Si deposition has been analyzed by Hierlemann et. al. Computers will prob-
ably have to advance by another couple of orders of magnitude before surface kinetics can be rou-
tinely determined by these methods. Until then, a semiempirical approach is often used. This consists
of using chemical intuition to postulate some chemical pathways. One then looks at the thermody-
namics of the system and rejects the paths that are not energetically favorable. Careful experiments
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.