Page 228 - Separation process engineering
P. 228
0.434 mole frac cumene and is a saturated liquid. F = 1.0 kmol/h. Feed stage is number 10 above the
partial reboiler, and there are 19 equilibrium stages plus a partial reboiler. A total condenser is
used. p = 101.3 kPa. Relative volatilities: α = 2.25, α = 1.0, α = 0.21. L/D = 1.0.
ben tol cum
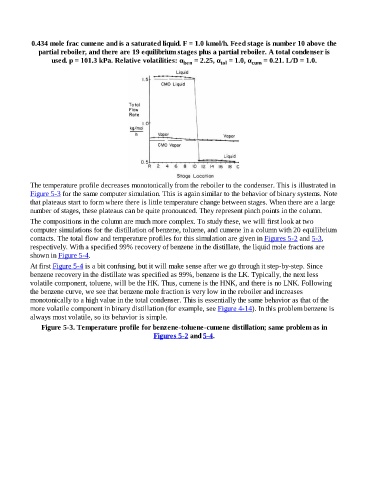
The temperature profile decreases monotonically from the reboiler to the condenser. This is illustrated in
Figure 5-3 for the same computer simulation. This is again similar to the behavior of binary systems. Note
that plateaus start to form where there is little temperature change between stages. When there are a large
number of stages, these plateaus can be quite pronounced. They represent pinch points in the column.
The compositions in the column are much more complex. To study these, we will first look at two
computer simulations for the distillation of benzene, toluene, and cumene in a column with 20 equilibrium
contacts. The total flow and temperature profiles for this simulation are given in Figures 5-2 and 5-3,
respectively. With a specified 99% recovery of benzene in the distillate, the liquid mole fractions are
shown in Figure 5-4.
At first Figure 5-4 is a bit confusing, but it will make sense after we go through it step-by-step. Since
benzene recovery in the distillate was specified as 99%, benzene is the LK. Typically, the next less
volatile component, toluene, will be the HK. Thus, cumene is the HNK, and there is no LNK. Following
the benzene curve, we see that benzene mole fraction is very low in the reboiler and increases
monotonically to a high value in the total condenser. This is essentially the same behavior as that of the
more volatile component in binary distillation (for example, see Figure 4-14). In this problem benzene is
always most volatile, so its behavior is simple.
Figure 5-3. Temperature profile for benzene-toluene-cumene distillation; same problem as in
Figures 5-2 and 5-4.