Page 168 - Separation process principles 2
P. 168
4.5 Ternary Liquid-Liquid Systems 133
Furfural
(C)
Mass fraction furfural
(a)
-
m 0.6 -
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 '0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Mass fraction furfural Mass fraction glycol in raffinate
(b) (c)
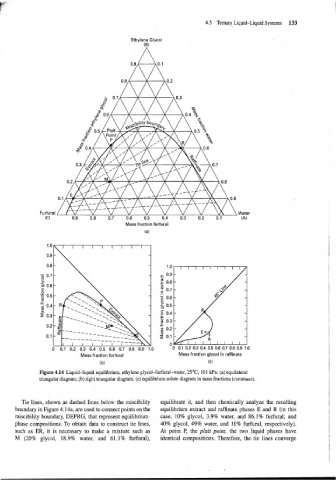
Figure 4.14 Liquid-liquid equilibrium, ethylene glycol-furfural-water, 25°C 101 kPa: (a) equilateral
triangular diagram; (b) right triangular diagram; (c) equilibrium solute diagram in mass fractions (continues).
Tie lines, shown as dashed lines below the miscibility equilibrate it, and then chemically analyze the resulting
boundary in Figure 4.14a, are used to connect points on the equilibrium extract and raffinate phases E and R (in this
miscibility boundary, DEPRG, that represent equilibrium- case, 10% glycol, 3.9% water, and 86.1 % furfural; and
phase compositions. To obtain data to construct tie lines, 40% glycol, 49% water, and 11% furfural, respectively).
such as ER, it is necessary to make a mixture such as At point P, the plait point, the two liquid phases have
M (20% glycol, 18.9% water, and 61.1% furfural), identical compositions. Therefore, the tie lines converge