Page 92 - Synthetic Fuels Handbook
P. 92
FUELS FROM PETROLEUM AND HEAVY OIL 79
Hydrocracking. Hydrocracking is similar to catalytic cracking, with hydrogenation
superimposed and with the reactions taking place either simultaneously or sequentially.
Hydrocracking was initially used to upgrade low-value distillate feedstocks, such as cycle
oils (high aromatic products from a catalytic cracker which usually are not recycled to
extinction for economic reasons), thermal and coker gas oils, and heavy-cracked and
straight-run naphtha. These feedstocks are difficult to process by either catalytic cracking
or reforming, since they are characterized usually by a high polycyclic aromatic content
and/or by high concentrations of the two principal catalyst poisons—sulfur and nitrogen
compounds.
A comparison of hydrocracking with hydrotreating is useful in assessing the parts played
by these two processes in refinery operations. Hydrotreating of distillates may be defined
simply as the removal of nitrogen—sulfur and oxygen-containing compounds by selective
hydrogenation. The hydrotreating catalysts are usually cobalt plus molybdenum or nickel
plus molybdenum (in the sulfide) form impregnated on an alumina base. The hydrotreated
operating conditions are such that appreciable hydrogenation of aromatics will not occur—
1000 to 2000 psi hydrogen and about 370°C (698°F). The desulfurization reactions are
usually accompanied by small amounts of hydrogenation and hydrocracking.
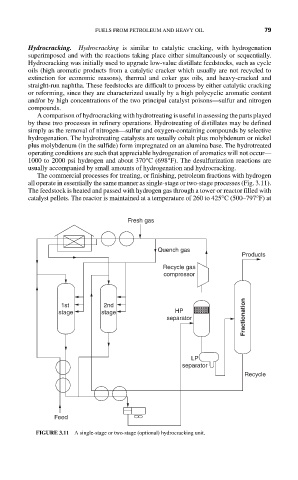
The commercial processes for treating, or finishing, petroleum fractions with hydrogen
all operate in essentially the same manner as single-stage or two-stage processes (Fig. 3.11).
The feedstock is heated and passed with hydrogen gas through a tower or reactor filled with
catalyst pellets. The reactor is maintained at a temperature of 260 to 425°C (500–797°F) at
Fresh gas
Quench gas
Products
Recycle gas
compressor
Fractionation
1st 2nd
stage stage HP
separator
LP
separator
Recycle
Feed
FIGURE 3.11 A single-stage or two-stage (optional) hydrocracking unit.