Page 95 - Synthetic Fuels Handbook
P. 95
82 CHAPTER THREE
The composition of a reforming catalyst is dictated by the composition of the feedstock
and the desired reformate. The catalysts used are principally molybdena-alumina, chromia-
alumina, or platinum on a silica-alumina or alumina base. The nonplatinum catalysts are
widely used in regenerative process for feeds containing, for example, sulfur, which poisons
platinum catalysts, although pretreatment processes (e.g., hydrodesulfurization) may permit
platinum catalysts to be employed.
The purpose of platinum on the catalyst is to promote dehydrogenation and hydrogena-
tion reactions, that is, the production of aromatics, participation in hydrocracking, and rapid
hydrogenation of carbon-forming precursors. For the catalyst to have an activity for isomeri-
zation of both paraffins and naphthenes—the initial cracking step of hydrocracking—and to
participate in paraffin dehydrocyclization, it must have an acid activity. The balance between
these two activities is most important in a reforming catalyst. In fact, in the production of
aromatics from cyclic saturated materials (naphthenes), it is important that hydrocracking
be minimized to avoid loss of the desired product and, thus, the catalytic activity must be
moderated relative to the case of gasoline production from a paraffinic feed, where dehy-
drocyclization and hydrocracking play an important part.
3.3.7 Isomerization Processes
Catalytic reforming processes provide high-octane constituents in the heavier gasoline frac-
tion but the normal paraffin components of the lighter gasoline fraction, especially butanes,
pentanes, and hexanes, have poor octane ratings. The conversion of these normal paraffins
to their isomers (isomerization) yields gasoline components of high octane rating in this
lower boiling range. Conversion is obtained in the presence of a catalyst (aluminum chlo-
ride activated with hydrochloric acid), and it is essential to inhibit side reactions such as
cracking and olefin formation.
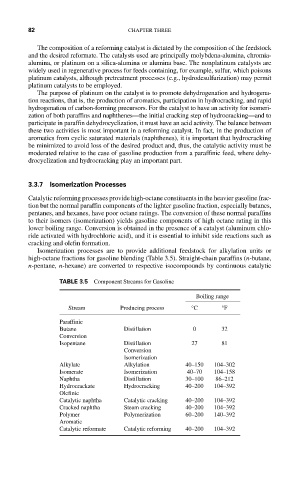
Isomerization processes are to provide additional feedstock for alkylation units or
high-octane fractions for gasoline blending (Table 3.5). Straight-chain paraffins (n-butane,
n-pentane, n-hexane) are converted to respective isocompounds by continuous catalytic
TABLE 3.5 Component Streams for Gasoline
Boiling range
Stream Producing process °C °F
Paraffinic
Butane Distillation 0 32
Conversion
Isopentane Distillation 27 81
Conversion
Isomerization
Alkylate Alkylation 40–150 104–302
Isomerate Isomerization 40–70 104–158
Naphtha Distillation 30–100 86–212
Hydrocrackate Hydrocracking 40–200 104–392
Olefinic
Catalytic naphtha Catalytic cracking 40–200 104–392
Cracked naphtha Steam cracking 40–200 104–392
Polymer Polymerization 60–200 140–392
Aromatic
Catalytic reformate Catalytic reforming 40–200 104–392