Page 96 - Synthetic Fuels Handbook
P. 96
FUELS FROM PETROLEUM AND HEAVY OIL 83
(aluminum chloride, noble metals) processes. Natural gasoline or light straight-run gaso-
line can provide feed by first fractionating as a preparatory step. High volumetric yields
(>95 percent) and 40 to 60 percent conversion per pass are characteristic of the isomeriza-
tion reaction.
Aluminum chloride was the first catalyst used to isomerize butane, pentane, and hex-
ane. Since then, supported metal catalysts have been developed for use in high-temperature
processes which operate in the range 370 to 480°C (698–896°F) and 300 to 750 psi
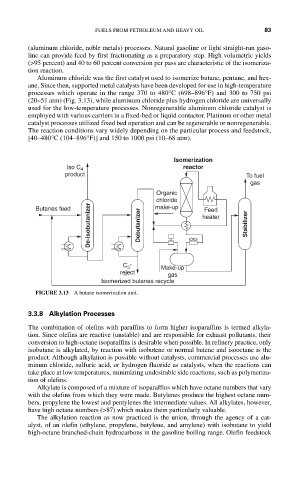
(20–51 atm) (Fig. 3.13), while aluminum chloride plus hydrogen chloride are universally
used for the low-temperature processes. Nonregenerable aluminum chloride catalyst is
employed with various carriers in a fixed-bed or liquid contactor. Platinum or other metal
catalyst processes utilized fixed bed operation and can be regenerable or nonregenerable.
The reaction conditions vary widely depending on the particular process and feedstock,
[40–480°C (104–896°F)] and 150 to 1000 psi (10–68 atm).
Isomerization
Iso C 4 reactor
product To fuel
gas
Organic
chloride
De-isobutanizer Debutanizer Stabilizer
Butanes feed make-up heater
Feed
C + Make-up
5
reject gas
Isomerized butanes recycle
FIGURE 3.13 A butane isomerization unit.
3.3.8 Alkylation Processes
The combination of olefins with paraffins to form higher isoparaffins is termed alkyla-
tion. Since olefins are reactive (unstable) and are responsible for exhaust pollutants, their
conversion to high-octane isoparaffins is desirable when possible. In refinery practice, only
isobutane is alkylated, by reaction with isobutene or normal butene and isooctane is the
product. Although alkylation is possible without catalysts, commercial processes use alu-
minum chloride, sulfuric acid, or hydrogen fluoride as catalysts, when the reactions can
take place at low temperatures, minimizing undesirable side reactions, such as polymeriza-
tion of olefins.
Alkylate is composed of a mixture of isoparaffins which have octane numbers that vary
with the olefins from which they were made. Butylenes produce the highest octane num-
bers, propylene the lowest and pentylenes the intermediate values. All alkylates, however,
have high octane numbers (>87) which makes them particularly valuable.
The alkylation reaction as now practiced is the union, through the agency of a cat-
alyst, of an olefin (ethylene, propylene, butylene, and amylene) with isobutane to yield
high-octane branched-chain hydrocarbons in the gasoline boiling range. Olefin feedstock