Page 84 - The Geological Interpretation of Well Logs
P. 84
- THE GEOLOGICAL INTERPRETATION OF WELL LOGS -
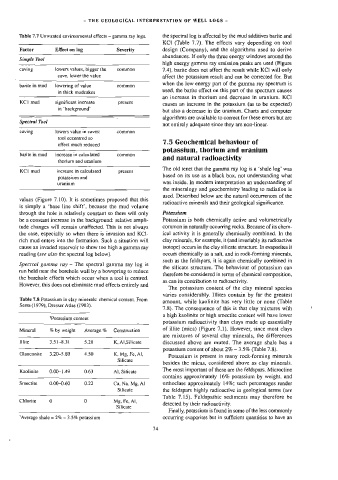
Table 7.7 Unwanted environmental effects — gamma ray logs. the spectral log is affected by the mud additives barite and
KCl (Table 7.7). The effects vary depending on tool
Factor Effect on log Severity design (Company), and the algorithms used to derive
abundances. If only the three energy windows around the
Simple Toot
high energy gamma ray emission peaks are used (Figure
caving lowers values, bigger the common 7.4), barite does not affect the result while KCI will only
cave, lower the value affect the potassium result and can be corrected for. But
when the low energy part of the gamma ray spectrum is
bariteinmud lowering of value common
used, the barite effect on this part of the spectrum causes
in thick mudcakes
an increase in thorium and decrease in uranium. KCl
KC] mud significant increase present
causes an increase in the potassium (as to be expected)
in “background”
but also a decrease in the uranium. Charts and computer
algorithms are available to correct for these errors but are
Spectral Tool
not entirely adequate since they are non-linear.
caving lowers value in caves: cammon
tool eccentred so
7.5 Geochemical behaviour of
effect much reduced
potassium, thorium and uranium
barite in mud —_ increase in calculated common
and natural radioactivity
thorium and uranium
The old tenet that the gamma ray log is a ‘shale log’ was
KCI mud increase in calculated present
based on its use as a black box, not understanding what
potassium and
uranium was inside. In modern interpretation an understanding of
the mineralogy and geochemistry leading to radiation is
used. Described below are the natural occurrences of the
values (Figure 7.10). It is sometimes proposed that this
radioactive minerals and their geological significance.
is simply a “base line shift’, because the mud volume
through the hole is relatively constant so there will only Potassium
be a constant increase in the background: relative ampli- Potassium is both chemically active and volumetrically
tude changes will remain unaffected. This is not always common in naturally occurring rocks. Because of its chem-
the case, especially so when there is invasion and KCI- ical activity it is generally chemically combined. In the
rich mud enters into the formation. Such a situation will clay minerals, for example, it (and invariably its radioactive
cause an invaded reservoir to show too high a gamma ray isotope) occurs in the clay silicate structure. In evaporites it
Treading (see afso the spectral log below). occurs chemically as a salt, and in rock-forming minerals,
such as the feldspars, it is again chemically combined in
Spectral gamma ray — The spectral gamma ray log is
the silicate structure. The behaviour of potassium can
run held near the borehole wall by a bowspring to reduce
therefore be considered in terms of chemical composition,
the borehole effects which occur when a tool is centred.
as can its contribution to radioactivity.
However, this does not eliminate mud effects entirely and
The potassium content of the clay mineral species
varies considerably. lites contain by far the greatest
Table 7.8 Potassium in clay minerals: chemical content. From
amount, while kaolinite has very little or none (Table
Serra (1979), Dresser Atlas (1983).
7.8). The consequence of this is that clay mixtures with
a high kaolinite or high smectite content will have lower
"Potassium content
potassium radioactivity than clays made up essentially
of illite (mica) (Figure 7.1). However, since most clays
Mineral % by weight Average % Construction
are mixtures of several clay minerals, the differences
[lite 3.51-8.31 5.20 K, Al,Silicate discussed above are muted. The average shale has a
potassium content of about 2% — 3.5% (Table 7.8).
Glauconite 3.20-5.80 4.50 K, Mg, Fe, Al,
Potassium is present in many rock-forming minerals
Silicate
besides the micas, considered above as clay minerals.
The most important of these are the feldspars. Microcline
Kaolinite 0.00-1.49 0.63 Al, Silicate
contains approximately 16% potassium by weight, and
Smectite 0.00-0.60 9.22 Ca, Na, Mg, Al orthoclase approximately 14%; such percentages render
Silicate the feldspars highly radioactive in geological terms (see
Table 7.15). Feldspathic sediments may therefore be
Chlorite 0 0 Mg, Fe, Al,
detected by their radioactivity.
Silicate
Finally, potassium is found in some of the less commonly
‘Average shale = 2% —- 3.5% potassium occurring evaporites but in sufficient quantities to have an
74