Page 85 - The Geological Interpretation of Well Logs
P. 85
- FHE GAMMA RAY AND SPECTRAL GAMMA RAY LOGS -
Table 7.9 Potassium content of evaporites. conditions, but is less common) which contains on aver-
age 0.6 g/ml of uranium in solution. However, it is
Species Formula % Potassium Typical gamma suggested that most (around 90%) uranium in rivers is
by weight* ray value API’ actually carried attached (loosely?) to clay particles and
not in solution (Durrance, 1986). This is suggested
Sylvite KCl 52.5 500 because suspended river sediment contains approxi-
Camallite KC1.MgC1,(H,0), 14.1 200 mately 3ppm of uranium, while the bedload sediments
Polyhalite K,SO,MgSO, 12.9 190 have much lower values, Sea water, on average contains
about 3ppb of dissolved uranium.
*Serma et ail., 1980
From river or especially sea water, uranium passes
"Serra, 1979.
into sediments in three principal ways (Serra, 1979): 1,
important effect on the radioactivity (Table 7.9). In these chemical precipitation in acid (pH 2.5-4.0), reducing (rH
salts there is between 10% and 50% potassium by weight. Q-0.4) environments: 2, adsorption by organic matter,
When it is considered that the average shale contains only or living plants and animals: 3, chemical reaction in
2% — 3.5% potassium, the very strong radioactivity of these phosphorites (phosphate rich rocks).
potassium evapontes is understandable (Table 7.9, Figure The extremely acid, reducing conditions required for the
7.23). direct chemical precipitation of uranium (pH 2.5-4.0, rH
0-0.4) are found in few natural environments. They do
Uranium occur, however, in stagnant, anoxic waters with a relatively
Acid igneous rocks on average contain 4.65ppm of slow rate of sediment deposition, which typically produce
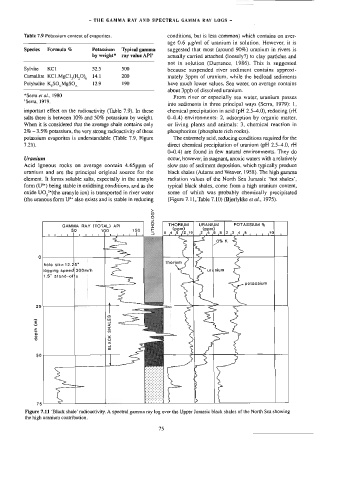
uranium and are the principal original source for the black shales (Adams and Weaver, 1958). The high gamma
element. It forms soluble salts, especially in the uranyle radiation values of the North Sea Jurassic ‘hot shales’,
form (U%) being stable in oxidising conditions, and as the typical black shales, come from a high uranium content,
oxide UO,*(the uranyle ion) is wansported in river water some of which was probably chemically precipitated
(the uranous form U** also exists and is stable in reducing (Figure 7.11, Table 7.10) (Bjgrlykke e ai., 1975).
>
oO
o
GAMMA RAY (TOTAL) API 7 monn URANIUM POTASSIUM %
50 100 150 5 ep Te 16 fP2™) 8 |2
18 OB foe oP Biase, th
=> > O% K %
a
0 =
hole size 12.25” tro
legging speed] 300m/h uranium
1.5" siand-offs
et ae Potassium
=o
t
25
wn
€ 4
= =<
: 5
a
o x
20 oO .
<=
a
m
50
q
75
|
Figure 7.11 ‘Black shale’ radioactivity. A spectral gamma ray log over the Upper Jurassic black shales of the North Sea showing
the high uranium contribution.
75