Page 90 - The Geological Interpretation of Well Logs
P. 90
- THE GEOLOGICAL INTERPRETATION OF WELL LOGS -
There are many examples of radioactive sandstones Neglecting radioactive sandstone intervals as being
that may be quoted. The fine-grained mica sands of the shales means missing essential reservoir. The fact that
North Sea (Nyberg e a/., 1978) are a typical, well-known only potassium should be causing detrital mineral
example (Figure 7.17). Some marine sands contain radioactivity in sandstones (e.g. Table 7.15) is used in the
glauconite and, if the concentrations are sufficiently high, interpretation of the spectral gamma ray log to separate
render the sands radioactive (Figure 7.18). In fact shale radioactivity from detrital grain radioactivity (see
tadioactive sandstones are far more common than real- below, ‘Quantitative uses of the spectral gamma ray’).
ized. Arkoses are radioactive by definition (Table 7.16).
Radioactivity in carbonates
Thorium, as previously described, is present in heavy-
Carbonates in their pure state are not radioactive and this
mineral suites. Placer silts (concentrations of heavy
aids their identification (Figure 7.1). Nonetheless, in
minerals) are frequently radioactive, producing a spiky
certain facies, carbonates contain organic matter and this
aspect to the gamma ray log (Figure 7.19). However, this
is frequently radioactive due to uranium. This is certainly
is the only case, and in general detntal grain radioactivity
the case in the example given (Figure 7.20) and it is pro-
is caused by potassium (Table 7.16).
posed (Hassan, 1973) that pure carbonate radioactivity is
For sandstone reservoir studies, identifying clay as
due only to uranium. Shaly carbonates wil] show the
opposed to non-clay radioactive elements is important.
presence of potassium and thorium.
LITHOLOGY
88
J D
c
O°
wi
GAMMA RAY API l > on o
cs w o
|=
tr
0 50 100 =—180 wo x Oo ;w
|=
o~
0
1
}
quartz 65%
feldspar 4% °
clay 15% Z
medium sand (350”) w
°
nN on quartz 50% a
zflc
o\8
clay 20% Se
(m) pyrite 5% wl a s
:
7
depth vy. fine sand (100%) |o
5s 4
feldspar 5% € 8
quartz 55%
clay 15%
2
coarse sand (10004)
a
garnet 10%
og/6
«2
oa) S
S2\5
0 50 100 150
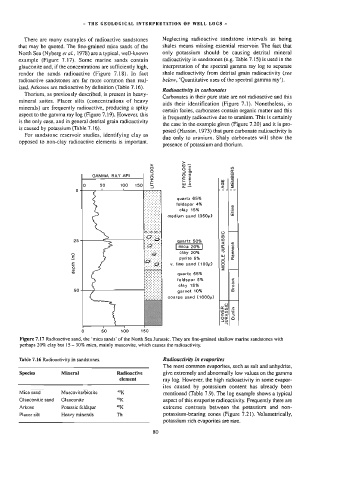
Figure 7.17 Radioactive sand, the ‘mica sands’ of the North Sea Jurassic. They are fine-grained shallow marine sandstones with
perhaps 20% clay but 15 - 30% mica, mainly muscovite, which causes the radioactivity.
Table 7.16 Radioactivity in sandstones. Radioactivity in evaporites
The most common evaporites, such as salt and anhydrite,
Species Mineral Radioactive give extremely and abnormally low values on the gamma
element ray log. However, the high radioactivity in some evapor-
ites caused by potassium content has already been
Mica sand Muscovite/biotite “OK mentioned (Table 7.9), The log example shows a typical
Glauconitic sand Glauconite OK aspect of this evaporite radioactivity. Frequently there are
Arkose Potassic feldspar OK extreme contrasts between the potassium and non-
Placer silt Heavy minerals Th potassium-bearing zones (Figure 7.21). Volumetrically,
potassium rich evaporites are rare.
80