Page 86 - The Geological Interpretation of Well Logs
P. 86
- THE GEOLOGICAL INTERPRETATION OF WELL LOGS -
Table 7.10 The abundance of the radioactive elements and Table 7.11 Average weight (%) of organic matter in sediments
their relative contributions to the overall radioactivity of the (from Shaw, 1986).
black shale example of Figure 7,11 (values calculated for the
peak at 40m). Sediment Average weight %
Element Content *gamma ray % gamma ray
Shales 2.90
API equivalent = yalue
Carbonates 0.29
Uranium ~—=sL] ppm 89.0API 41.0% Sandstones 0.05
Thorium = =|8 ppm 70.7 API 32.6%
3 4
® ’
Potassium 3.5% 57.1 API 26.4% = °
s VA
*using the multipliers given in the text (section 7.4) aa ° LL. °
5 *
z ° 3s
ao.
fo
Probably a more common way of introducing uranium < ee
into sediments is in association with organic matter. It 8 °
s
o g!' jo i“ Q
has been established experimentally that carbonaceous
ciently, especially over the range of pH 3.5-6.0 (acidic) Oo 4 ° °
<
=
materia] can extract uranium from solution very effi-
6
o|
re
(Durrance, 1986). Organic-rich shales often (but not 4
uranium ppm
always) contain large amounts of syngenetic uranium (i.e.
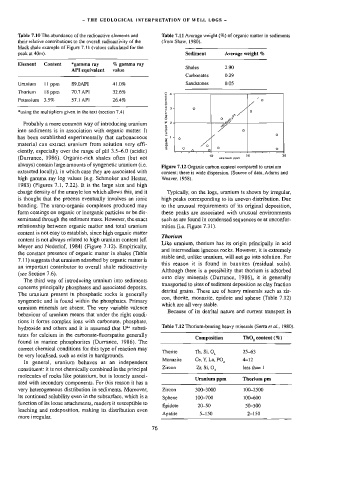
Figure 7.12 Organic carbon content compared to uranium
extracted locally), in which case they are associated with content: there is wide dispersion. (Source of data, Adams and
high gamma ray log values (e.g. Schmoker and Hester, Weaver, 1958).
1983) (Figures 7.1, 7.22). It is the large size and high
charge density of the uranyle ion which allows this, and it Typically, on the logs, uranium is shown by irregular,
is thought that the process eventually involves an ionic high peaks corresponding to its uneven distribution. Due
bonding. The urano-organic complexes produced may to the unusual requirements of its original deposition,
form coatings on organic or inorganic particles or be dis- these peaks are associated with unusual environments
seminated through the sediment mass. However, the exact such as are found in condensed sequences or at unconfor-
relationship between organic matter and total] uranium mities (i.e. Figure 7.31).
content is not easy to establish, since high organic matter
Thorium
content is not always related to high uranium content (cf.
Like uranium, thorium has its origin principally in acid
Meyer and Nederlof, 1984) (Figure 7.12). Empirically,
and intermediate igneous rocks. However, it is extremely
the constant presence of organic matter in shales (Table
stable and, unlike uranium, will not go into solution. For
7.11) suggests that uranium adsorbed by organic matter is
this reason it is found in bauxites (residual soils).
an important contributor to overall shale radioactivity
Although there is a possibility that thorium is adsorbed
(see Section 7.6).
onto clay minerals (Durrance, 1986), it is generally
The third way of introducing uranium into sediments
transported to sites of sediment deposition as clay fraction
concerns principally phosphates and associated deposits.
detrital grains. These are of heavy minerals such as zir-
The uranium present in phosphatic rocks is generally
con, thorite, monazite, epidote and sphene (Table 7.12)
syngenetic and is found within the phosphates. Primary
which are all very stable.
uranium minerals are absent. The very variable valence
Because of its detrital nature and current transport in
behaviour of uranium means that under the right condi-
tions it forms complex ions with carbonate, phosphate,
hydroxide and others and it is assumed that U* substi- Table 7.12 Thorium-bearing heavy minerals (Serra et al., 1980).
tutes for calcium in the carbonate-fluorapatite generally
Composition ThO, content (%)
found in marine phosphorites (Durrance, 1986). The
correct chemical conditions for this type of reaction may
Thorite Th, Si, O, 25-63
be very localised, such as exist in hardgrounds.
Monazite Ce, Y, La, PO, 4-12
In general, uranium behaves as an independent
constituent: it is not chemically combined in the principal Zircon Zr, Si, O, less than |
molecules of rocks like potassium, but is loosely associ-
Uranium ppm Thorium pm
ated with secondary components. For this reason it has a
very heterogeneous distribution in sediments. Moreover, Zircon 300-3000 100-2500
its continued solubility even in the subsurface, which is a
Sphene 100-700 100-600
function of its loose attachments, renders it susceptible to
Epidote 20-50 50-500
leaching and redeposition, making its distribution even
Apatite 5-150 2-150
more irregular.
76