Page 352 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 352
P1: JDW
June 22, 2007
14:25
AT029-Manual
AT029-08
AT029-Manual-v7.cls
332 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
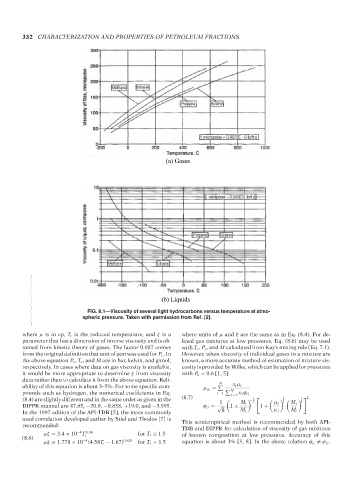
(a) Gases
(b) Liquids
FIG. 8.1—Viscosity of several light hydrocarbons versus temperature at atmo-
spheric pressure. Taken with permission from Ref. [2].
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
where μ is in cp, T r is the reduced temperature, and ξ is a where units of μ and ξ are the same as in Eq. (8.4). For de-
parameter that has a dimension of inverse viscosity and is ob- fined gas mixtures at low pressures, Eq. (8.6) may be used
tained from kinetic theory of gases. The factor 0.987 comes with T c , P c , and M calculated from Kay’s mixing rule (Eq. 7.1).
from the original definition that unit of atm was used for P c .In However, when viscosity of individual gases in a mixture are
the above equation P c , T c , and M are in bar, kelvin, and g/mol, known, a more accurate method of estimation of mixture vis-
respectively. In cases where data on gas viscosity is available, cosity is provided by Wilke, which can be applied for pressures
it would be more appropriate to determine ξ from viscosity with P r < 0.6 [1, 5]:
data rather than to calculate it from the above equation. Reli-
ability of this equation is about 3–5%. For some specific com- μ m = N x i μ i
pounds such as hydrogen, the numerical coefficients in Eq. i=1 N x j φ ij
j=1
(8.4) are slightly different and in the same order as given in the (8.7) 1 − 1 2 1 1 4 2
2
DIPPR manual are 47.65, −20.0, −0.858, +19.0, and −3.995. φ ij = √ 1 + M i 1 + μ i M j
In the 1997 edition of the API-TDB [5], the more commonly 8 M j μ j M i
used correlation developed earlier by Stiel and Thodos [7] is This semiempirical method is recommended by both API-
recommended:
TDB and DIPPR for calculation of viscosity of gas mixtures
−4
μξ = 3.4 × 10 T r 0.94 for T r ≤ 1.5 of known composition at low pressures. Accuracy of this
(8.6)
−4
μξ = 1.778 × 10 (4.58T r − 1.67) 0.625 for T r > 1.5 equation is about 3% [5, 8]. In the above relation φ ij = φ ji .
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT