Page 395 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 395
QC: IML/FFX
T1: IML
P1: IML/FFX
P2: IML/FFX
14:25
June 22, 2007
AT029-Manual-v7.cls
AT029-Manual
AT029-09
9. APPLICATIONS: PHASE EQUILIBRIUM CALCULATIONS 375
reversal and its understanding will help plan for more effi-
cient oil recovery processes [24]. Similarly, asphaltene depo-
sition negatively affects the EOR gas flooding projects [25].
Asphaltene precipitation may also occur during oil processing
in refineries or transportation in pipelines and causes major
problems by plugging pipes and catalysts pores [26, 27]. The
problem is more severe for heavy oils. Further information
on problems associated with asphaltene precipitation during
production, especially in the Middle East fields, is given by
Riazi et al. [28].
There are a number of models and theories that are pro-
posed to describe mechanism of asphaltene formation [29].
Understanding of kinetics of asphaltene formation is much
more difficult than wax formation. There is no universally
accepted model for asphaltene formation; however, most re-
searchers agree on two models: (1) colloidal and (2) micellar.
Schematic of colloidal model is shown in Fig. 9.7. The nature
and shape of the resulting aggregates will determine their
effect on the behavior of the petroleum fluid [30, 31]. In
this model, asphaltene particles come together to form larger
molecules (irreversible aggregation), which grow in size. Ac-
cording to this model the surface of asphaltene molecules
must be fully covered by resin molecules. For this reason
when concentration of resin exceeds from a certain level, rate
of asphaltene deposition decreases even if its concentration
is high. Because of this it is often possible that an oil with
higher asphaltene content results in less precipitation due to
high resin content in comparison with an oil with lower as-
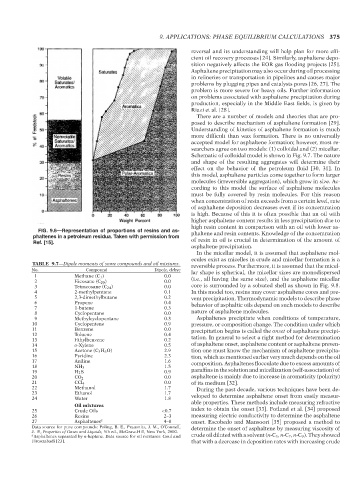
FIG. 9.6—Representation of proportions of resins and as- phaltene and resin contents. Knowledge of the concentration
phaltenes in a petroleum residua. Taken with permission from of resin in oil is crucial in determination of the amount of
Ref. [15].
asphaltene precipitation.
In the micellar model, it is assumed that asphaltene mol-
ecules exist as micelles in crude and micellar formation is a
TABLE 9.7—Dipole moments of some compounds and oil mixtures. reversible process. Furthermore, it is assumed that the micel-
No. Compound Dipole, debye
lar shape is spherical, the micellar sizes are monodispersed
1 Methane (C 1 ) 0.0
2 Eicosane (C 20 ) 0.0 (i.e., all having the same size), and the asphaltene micellar
3 Tetracosane (C 24 ) 0.0 core is surrounded by a solvated shell as shown in Fig. 9.8.
4 2-methylpentane 0.1 In this model too, resins may cover asphaltene cores and pre-
5 2,3-dimethylbutane 0.2 vent precipitation. Thermodynamic models to describe phase
6 Propene 0.4 behavior of asphaltic oils depend on such models to describe
7 1-butene 0.3
8 Cyclopentane 0.0 nature of asphaltene molecules.
9 Methylcyclopentane 0.3 Asphaltenes precipitate when conditions of temperature,
10 Cyclopentene 0.9 pressure, or composition change. The condition under which
11 Benzene 0.0 precipitation begins is called the onset of asphaltene precipi-
12 Toluene 0.4
13 Ethylbenzene 0.2 tation. In general to select a right method for determination
14 o-Xylene 0.5 of asphaltene onset, asphaltene content or asphaltene preven-
15 Acetone (C 3 H 6 O) 2.9 tion one must know the mechanism of asphaltene precipita-
16 Pyridine 2.3 tion, which as mentioned earlier very much depends on the oil
17 Aniline 1.6 composition. Asphaltenes flocculate due to excess amounts of
18 NH 3 1.5
19 H 2 S 0.9 paraffins in the solution and micellization (self-association) of
20 CO 2 0.0 asphaltene is mainly due to increase in aromaticity (polarity)
21 CCl 4 0.0 of its medium [32].
22 Methanol 1.7 During the past decade, various techniques have been de-
23 Ethanol 1.7
24 Water 1.8 veloped to determine asphaltene onset from easily measur-
able properties. These methods include measuring refractive
Oil mixtures
25 Crude Oils <0.7 index to obtain the onset [33]. Fotland et al. [34] proposed
26 Resins 2–3 measuring electric conductivity to determine the asphaltene
27 Asphaltenes a 4–8 onset. Escobedo and Mansoori [35] proposed a method to
Data source for pure compounds: Poling, B. E., Prausnitz, J. M., O’Connell, determine the onset of asphaltene by measuring viscosity of
J. P., Properties of Gases and Liquids, 5th ed., McGraw-Hill, New York, 2000.
a Asphaltenes separated by n-heptane. Data source for oil mixtures: Goul and crude oil diluted with a solvent (n-C 5 , n-C 7 , n-C 9 ). They showed
Firoozabadi [23]. that with a decrease in deposition rates with increasing crude
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---