Page 397 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 397
QC: IML/FFX
T1: IML
P2: IML/FFX
P1: IML/FFX
June 22, 2007
14:25
AT029-Manual-v7.cls
AT029-09
AT029-Manual
9. APPLICATIONS: PHASE EQUILIBRIUM CALCULATIONS 377
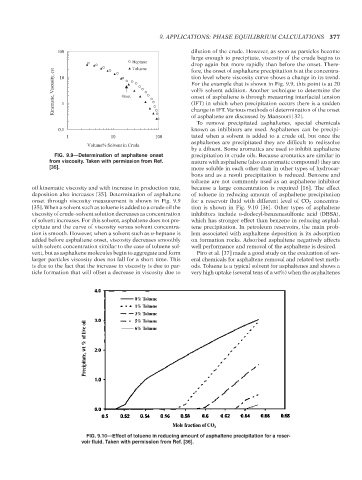
dilution of the crude. However, as soon as particles become
100
large enough to precipitate, viscosity of the crude begins to
Heptane
drop again but more rapidly than before the onset. There-
Toluene
Kinematic, Viscusity, cst 1 Onset For the example that is shown in Fig. 9.9, this point is at 20
fore, the onset of asphaltene precipitation is at the concentra-
tion level where viscosity curve shows a change in its trend.
10
vol% solvent addition. Another technique to determine the
onset of asphaltene is through measuring interfacial tension
(IFT) in which when precipitation occurs there is a sudden
change in IFT. Various methods of determination of the onset
of asphaltene are discussed by Mansoori [32].
To remove precipitated asphaltenes, special chemicals
0.1 known as inhibitors are used. Asphaltenes can be precipi-
1 10 100 tated when a solvent is added to a crude oil, but once the
asphaltenes are precipitated they are difficult to redissolve
Volume% Solvent in Crude
by a diluent. Some aromatics are used to inhibit asphaltene
FIG. 9.9—Determination of asphaltene onset precipitation in crude oils. Because aromatics are similar in
from viscosity. Taken with permission from Ref. nature with asphaltene (also an aromatic compound) they are
[35]. more soluble in each other than in other types of hydrocar-
bons and as a result precipitation is reduced. Benzene and
toluene are not commonly used as an asphaltene inhibitor
oil kinematic viscosity and with increase in production rate, because a large concentration is required [16]. The effect
deposition also increases [35]. Determination of asphaltene of toluene in reducing amount of asphaltene precipitation
onset through viscosity measurement is shown in Fig. 9.9 for a reservoir fluid with different level of CO 2 concentra-
[35]. When a solvent such as toluene is added to a crude oil the tion is shown in Fig. 9.10 [36]. Other types of asphaltene
viscosity of crude–solvent solution decreases as concentration inhibitors include n-dodecyl-benzenesulfonic acid (DBSA),
of solvent increases. For this solvent, asphaltene does not pre- which has stronger effect than benzene in reducing asphal-
cipitate and the curve of viscosity versus solvent concentra- tene precipitation. In petroleum reservoirs, the main prob-
tion is smooth. However, when a solvent such as n-heptane is lem associated with asphaltene deposition is its adsorption
added before asphaltene onset, viscosity decreases smoothly on formation rocks. Adsorbed asphaltene negatively affects
with solvent concentration similar to the case of toluene sol- well performance and removal of the asphaltene is desired.
vent, but as asphaltene molecules begin to aggregate and form Piro et al. [37] made a good study on the evaluation of sev-
larger particles viscosity does not fall for a short time. This eral chemicals for asphaltene removal and related test meth-
is due to the fact that the increase in viscosity is due to par- ods. Toluene is a typical solvent for asphaltenes and shows a
ticle formation that will offset a decrease in viscosity due to very high uptake (several tens of a wt%) when the asphaltenes
FIG. 9.10—Effect of toluene in reducing amount of asphaltene precipitation for a reser-
voir fluid. Taken with permission from Ref. [36].
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT