Page 177 - Separation process principles 2
P. 177
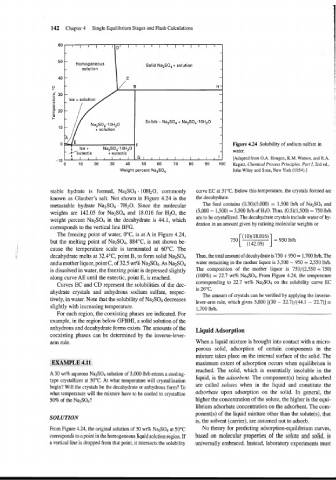
142 Chapter 4 Single Equilibrium Stages and Flash Calculations
60 l I I I I I D I I I I I I I I I I I I I
- -
-
50 - Homogeneous Solid Na2S04 + solution
- solution -
- -
40 C
-
9 H -
-
-
-
-
Solids - Na2S04 + Na2S0,.IOH,0
-
-
-
F Figure 4.24 Solubility of sodium sulfate in
lce + Na2S04~10H20
- Heutectic + eutectic - water.
G I I I I I I I I I I ~ [Adapted from O.A. Hougen, K.M. Watson, and R.A.
0 10 20 30 40 50 60 70 80 90 100 Ragatz, Chemical Process Principles. Part I, 2nd ed.,
Weight percent Na2S04 Joh11 Wiley and Sons, New York (1954).]
stable hydrate is formed, Na2S04. 10H20, commonly curve EC at 31°C. Below this temperature, the crystals formed are
known as Glauber's salt. Not shown in Figure 4.24 is the the decahydrate.
metastable hydrate Na2S04 . 7H20. Since the molecular The feed contains (0.30)(5,000) = 1,500 lb/h of Na2S04 and
(5,000 - 1,500) = 3,500 lb/h of H20. Thus, (0.5)(1,500) = 750 lb/h
weights are 142.05 for Na2S04 and 18.016 for H20, the
are to be crystallized. The decahydrate crystals include water of hy-
weight percent Na2S04 in the decahydrate is 44.1, which
dration in an amount given by ratioing molecular weights or
corresponds to the vertical line BFG.
The freezing point of water, O°C, is at A in Figure 4.24,
but the melting point of Na2S04, 884"C, is not shown be-
I cause the temperature scale is terminated at 60°C. The
I decahydrate melts at 32.4"C, point B, to form solid Na2S04 Thus, the total amount of decahydrate is 750 + 950 = 1,700 lblh. The
and a mother liquor, point C, of 32.5 wt% Na2S04. As Na2S04 water remaining in the mother liquor is 3,500 - 950 = 2,550 lbih.
is dissolved in water, the freezing point is depressed slightly The composition of the mother liquor is 750/(2,550 + 750)
along curve AE until the eutectic, point E, is reached. (100%) = 22.7 wt% Na4S04. From Figure 4.24, the temperature
Curves EC and CD represent the solubilities of the dec- corresponding to 22.7 wt% Na2S04 on the solubility curve EC
is 26°C.
ahydrate crystals and anhydrous sodium sulfate, respec-
The amount of crystals can be verified by applying the inverse-
tively, in water. Note that the solubility of Na2S04 decreases
lever-arm rule, which gives 5,000 [(30 - 22.7)/(44.1 - 22.7)] =
slightly with increasing temperature. 1,700 lbih.
For each region, the coexisting phases are indicated. For
example, in the region below GFBHI, a solid solution of the
anhydrous and decahydrate forms exists. The amounts of the
Liquid Adsorption
coexisting phases can be determined by the inverse-lever-
arm rule. When a liquid mixture is brought into contact with a micro-
porous solid, adsorption of certain components in the
mixture takes place on the internal surface of the solid. The
maximum extent of adsorption occurs when equilibrium is
reached. The solid, which is essentially insoluble in the
A 30 wt% aqueous Na2S04 solution of 5,000 lbih enters a cooling-
type crystallizer at 50°C. At what temperature will crystallization liquid, is the adsorbent. The component(s) being adsorbed
begin? Will the crystals be the decahydrate or anhydrous form? To are called solutes when in the liquid and constitute the
what temperature will the mixture have to be cooled to crystallize adsorbate upon adsorption on the solid. In general, the
50% of the Na2S04? higher the concentration of the solute, the higher is the equi-
librium adsorbate concentration on the adsorbent. The com-
ponent(~) of the liquid mixture other than the solute(s), that
SOLUTION
is, the solvent (carrier), are assumed not to adsorb.
From Figure 4.24, the original solution of 30 wt% Na2S04 at 50°C No theory for predicting adsorption-equilibrium curves,
corresponds to a point in the homogeneous liquid solution region. If based on molecular properties of the solute and solid, is
a vertical line is dropped from that point, it intersects the solubility universally embraced. Instead, laboratory experiments must